What is nucleotide ? Defination, Types, structure, Functions
The nucleic acid molecule is a long-chain polymer composed of monomeric units called nucleotides. Each nucleotide consists of a nucleoside and a phosphate group. Each nucleoside, in turn, consists of a sugar molecule and a nitrogenous base. The sugar is deoxyribose in the case of DNA (deoxyribose nucleic acid) and ribose in the case of RNA (ribose nucleic acid).
What is a nucleotide? Definition, Types, structure, Functions:
What is a nucleotide?
Nucleotides are energy-rich organic compounds containing nitrogen (base) attached to a sugar and phosphate group. Nucleotides have a very important role in the metabolic processes of living organisms, as they are the building blocks of nucleic acids, substances that control all hereditary characteristics.
As we know there are two types of nucleic acids, Ribonucleic acid (RNA) and deoxyribonucleic acid (DNA) These are the two fundamental structures that hold the essential functions for metabolic activities and reproduction of organisms. The nucleotide sequence in the DNA or RNA encodes the structure of the proteins synthesized in the cell.
The nucleotide adenosine triphosphate (ATP) is the energy source for many metabolic processes of living organisms. They are also part of the structural composition of enzymatic cofactors (inorganic substances essential for the functioning of enzymes) and nucleic acids and metabolic processes.
The nitrogenous bases are organic heterocyclic compounds made up of purines and pyrimidines. Purines such as (adenine and guanine) nicotinamide pyrrhotine and Pyrimidines such as (cytosine, thymine, and uracil).
Nucleosides are similar to nucleotides, except that they do not have the phosphate group. The nucleosides themselves rarely participate in cell metabolism. In certain vital metabolic processes, For example, nucleotides are carriers of intracellular chemical energy, such as adenosine triphosphate (adenosine triphosphate, ATP), guanosine triphosphate (guanosine triphosphate, GTP), it can also form the second messenger (cyclic adenosine monophosphate, cyclic guanosine monophosphate) in the process of cell signal transduction, and the formation of various coenzymes.
The nitrogenous bases are of two types :
(a) Purine: They are 9-membered double-ring nitrogen bases that possess nitrogen at 1′, 3,7, and 9′ positions e.g., adenine (A), guanine (G).
(b) Pyrimidine: They are 6-membered single-ring nitrogen bases that possess nitrogen at 1′ and 3 positions e.g. cytosine (C). thymine (T) and uracil (U). In DNA. thymine is present whereas, in RNA, uracil is present. In the pentose sugar, the nitrogenous base always attaches itself at a 1′ position by glycosidic bond by one of its nitrogen atoms (usually 1n pyrimidine and 9′ in purines). Phosphate is linked at 5 and 3 carbon atoms of pentose Sugar.
Definition of nucleotide:
A nucleotide is an organic compound that is made up of a nitrogenous base, sugar, and phosphoric acid. Nucleotides can act as monomers in nucleic acids (DNA or RNA), forming linear chains, or act as free molecules (as is the case with ATP).
Types of Nucleotide
Depending upon the type of pentose sugar present nucleotides are of two types of
(a) Ribonucleotides :
P+Ribose sugar (RS) + A – adenylic acid or adenosine monophosphate
P+RS +C- cacodylic acid or cytidine monophosphate
P+RS+G- guanylic acid or guanine monophosphate
P+RS + U- uridylic acid or uridine monophosphate
(b) Deoxyribonucleotides
P+ deoxyribose sugar (dRS) +A- deoxy adenylic acid
P+ deoxyribose sugar (M) +C polycytidylic acid
P+ deoxyribose sugar (M) +G- deoxyguanylic acid
P+deoxyribose sugar (M) +T deoxythymidylic acid
In eukaryotic cells the cells that have a defined cell nucleus, a nucleotide is found in the nucleus, whereas in prokaryotic cells (without a defined nucleus) nucleotide is found in the nucleoplasm. In molecular biology, nucleotides, which are the base units of DNA, contain the genetic information of the cell, and of RNA, which stores and transfers information to the ribosome for protein synthesis, part of what is called “central dogma”. Is: the pathway of information for the synthesis of proteins from DNA to RNA and then to ribosomes.
Higher nucleotides:
Nucleotides having more than one phosphate group are called higher nucleotides. They occur in the free state. The second and third phosphates of higher nucleotides (like ATP) are attached against forces of repulsion between similarly charged phosphate radicals. Hence the bonds attaching the second and third phosphates are high-energy bonds.
ATP is the most common energy carrier in cells and is generally called the “energy currency of the cell”. The third phosphate bond of ATP may release about 8.9 kcal of energy whereas the second energy-rich bond has energy equivalent to 6.5 kcal.
Nucleotides are also present in vitamins like nicotinamide and riboflavin in the form of coenzymes. Nicotinamide forms NAD and NADP. Similarly, flavin forms FAD and FADP. They are involved in respiration and photosynthesis by acting as coenzymes and enzymes.
Nucleotide Nomenclature
- The position of the atoms in a nucleotide is specified relative to the carbon atoms in the ribose or deoxyribose sugar.
- Purine or pyrimidine is located on carbon 1 of the sugar.
- The phosphate group is on carbon 5.
- The hydroxyl group is attached to carbon 3 of the sugar. It can be released in the form of water as a result of the formation of the phosphodiester bond.
- There may be an additional hydroxyl group attached to carbon 2 if the pentose is an r.
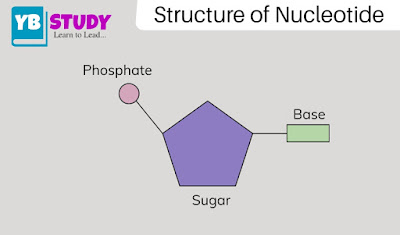
Nucleotide structure:
The nucleotide structure is composed of 3 parts: a nitrogenous base, a 5-carbon sugar, and a phosphate group. The polynucleotide chain is characterized by its directionality where the tail is called the 3 ‘end and the head is called the 5’ end.
1. Nitrogen base: The nitrogenous base is a ring structure containing nitrogen and maybe purine or pyrimidine. Purine can be adenine (A) or divided into guanine (G) and pyrimidine cytosine (C) or uracil (U).
2. Sugar: Polynucleotide chains have a central position of 5-carbon sugars that bind carbon (C) to one or more phosphate groups. Sugar is attached to its neighbors by a chain known as a phosphodiester bond. The carbon atoms in sugar are each called 1 ‘(one prime), 2’ (two prime), 3 ‘(three prime), 4’ (four prime, and 5 ‘(five prime). Nitrogen-based carbon 1 And phosphate group 5 ‘.
3. Phosphate group: Phosphate groups are 2 fused rings of carbon (C) and nitrogen (N) and one or more groups can be combined in a polynucleotide chain. The phosphate group is attached to the first carbon (5 ‘) of the sugar via a phosphodiester bond. Importantly, the structure of nucleotides is the basis of the structure of nucleic acids (DNA and RNA), therefore, they share the structure of a nitrogenous base, a 5-carbon sugar, and a phosphate group.
Nucleotides and nucleic acids
- The nucleotide is the base unit of nucleic acids (DNA and RNA) and consists of 4 nitrogenous bases, a 5-carbon sugar, and a phosphate group.
- Nitrogenous bases of nucleic acids are made up of 2 purines and 2 pyrimidines. Nitrogenous bases of deoxyribonucleic acid (DNA) are adenine (A), guanine (G), cytosine (C), and thymine (T). And ribonucleic acid (RNA) is adenine (A), guanine (G), cytosine (C), and uracil (U).
- The 5-carbon sugars in DNA are called deoxyribose and the sugars in RNA are called ribose.
Biological functions of nucleotides:
Structural function: they are the monomers that constitute nucleic acids, which are the biomolecules involved in the conservation, reproduction, and expression of genetic information.
Energetic function: if the nucleotide joins two or three phosphates, it forms the diphosphate or triphosphate nucleotides, such as ADP, and ATP, molecules that accumulate, transport, and give up energy.
Transfer of energy: Nucleotides, because their phosphate groups give them a high-energy bond, are preferred sources in cells for energy transfer. Nucleotides are in a stable state when they have only one phosphate group. Each additional phosphate group that a nucleotide possesses is in a more unstable state, and the phosphorus-phosphate bond tends, when broken by hydrolysis, to release the energy that binds it to the nucleotide.
Enzymatic Reactions: Cells have enzymes whose function is precisely to hydrolyze nucleotides to extract the energy potential stored in their bonds. For this reason, a triphosphate nucleotide is the most used source of energy in the cell. Of these, ATP (an adenine nucleotide with three phosphate groups rich in energy), is the central axis in cellular reactions for the transfer of the energy demanded. UTP (uracil + three phosphates) and GTP (guanine and three phosphates) also satisfy the energy demands of the cell in reactions with sugars and changes in protein structures, respectively.
Coenzymatic function: if the nucleotide is joined by a phosphate group to another substance, it gives non-nucleic nucleotides that act as coenzymes.
FAQs About Nucleotide
1. What is a nucleotide structure and function?
Answer: Nucleotides are the units that make up nucleic acids. Each nucleotide is a relatively complex molecule, made up of the union of three units: a monosaccharide (a pentose), a nitrogenous base, and one or more phosphate groups.
2. What is the structure of nucleotides?
Answer: RNA and DNA are polymers made up of long chains of nucleotides. A nucleotide is made up of a sugar molecule (ribose in RNA or deoxyribose in DNA) attached to a phosphate group and a nitrogenous base. The bases used in DNA are adenine (A), cytosine (C), guanine (G), and thymine (T).
3. Define Nucleotide
Answer: A nucleotide is an organic compound that is made up of a nitrogenous base, sugar, and phosphoric acid. Nucleotides can act as monomers in nucleic acids (DNA or RNA), forming linear chains, or act as free molecules (as is the case with ATP).
4. What are the types of nucleotides?
Answer: Each nucleotide has a fixed part (sugar and a phosphate group) and a nitrogenous base that distinguishes each type of nucleotide. In DNA there are four bases: adenine (A), cytosine (C), guanine (G), and thymine (T); and therefore four types of nucleotides.
5. What are the three components of a nucleotide?
Answer: What are nucleotides and what are their components?
- A nitrogenous base: for DNA formed by adenine (A), cytosine (C), guanine (G), and thymine (T).
- A sugar molecule: in the form of ribose in RNA or deoxyribose in DNA.
- A phosphate group: phosphoric acid.
6. What do 5 prime and 3 prime mean in DNA?
Answer: The chemical convention of naming the carbon atoms in the nucleotide pentose numerically gives it the 5 ‘end and 3’ end names (generally pronounced “five prime ends” and “three prime ends” respectively).