Class 12 chemistry chapter 15 Introduction to polymer chemistry solutions
Maharashtra state Chemistry Textbook Solutions for Class 12 are very important and crusial that helps the students in understanding the complex topics and helps them in the preparation of class 12 board examination as well as verious compititive entrance examinations also. Studying the answers to the questions in the Chemistry textbook will check your understanding of a particular topic and helps you determine your strengths and weaknesses.
Class 12 chemistry textbook Solutions for Class 12, Chemistry Chapter 15 Introduction to polymer chemistry maharashtra state board are provided here with simple step-by-step detailed explanations. These solutions for Introduction to polymer chemistry are very popular among Class 12 students for chemistry chapter 15 Introduction to polymer chemistry Solutions come handy for quickly completing your homework and preparing for exams. All questions and answers from the chemistry textbook Solutions Book of Class 12 chemistry Chapter 15 are provided here for you for free. You will also love the experience on ybstudy class 12 Solutions. All chemistry textbook Solutions. Solutions for class 12, These chemistry textbook solutions are prepared by Chemistry experts and are 100% accurate.
1. Choose the correct option from the given alternatives.
i. Nylon fibres are —-
A. Semisynthetic fibres
B. Polyamide fibres
C. Polyester fibres
D. Cellulose fibres
ii. Which of the following is naturally
occurring polymer ?
A. Telfon
B. Polyethylene
C. PVC
D. Protein
iii. Silk is a kind of —- fibre
A. Semisynthetic
B. Synthetic
C. Animal
D. Vegetable
iv. Dacron is another name of —-
A. Nylon 6
B. Orlon
C. Novolac
D. Terylene
v. Which of the following is made up of
polyamides ?
A. Dacron
B. Rayon
C. Nylon
D. Jute
vi. The number of carbon atoms present
in the ring of e – caprolactam is
A. Five
B. Two
C. Seven
D. Six
vii. Terylene is —-
A. Polyamide fibre
B. Polyester fibre
C. Vegetable fibre
D. Protein fibre
viii. PET is formed by —-
A. Addition
B. Condensation
C. Alkylation
D. Hydration
ix. Chemically pure cotton is —-
A. Acetate rayon
B. Viscose rayon
C. Cellulose nitrate
D. Cellulose
x. Teflon is chemically inert, due to
presence of ………..
A. C-H bond
B. C-F bond
C. H- bond
D. C=C bond
2. Answer the following in one sentence
each.
i. Identify ‘A’ and ‘B’ in the following
reaction…………
Answer : ———–
ii. Complete the following statements
a. Caprolactam is used to prepare——–
Answer : Nylon 6
b. Novolak is a copolymer of ——
— and ———
Answer : phenol and formaldehyde
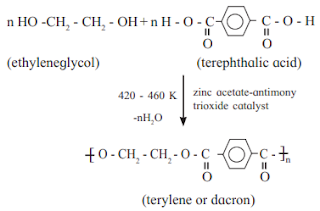
c. Terylene is ———-polymer of terephthalic acid and ethylene
glycol.
Answer : co-polymer
d. Benzoyl peroxide used in addtion polymerisation acts as ———-
Answer : initiator
e. Polyethene consists of
polymerised …………
Answer : ethylene (olefin) monomers.
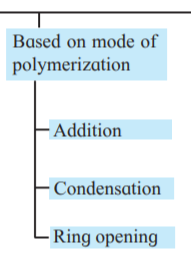
iii. Draw the flow chart diagram to show
classification of polymers based on
type of polymerisation.
Answer :
iv. Write examples of Addition polymers
and condensation polymers.
Answer : Common examples of addition polymerization are PVC, polyethene, Teflon etc.
Common examples of condensation polymerization are nylon, bakelite, silicon, etc
v. Name some chain growth polymers.
Answer : Many common polymers can be obtained by chain polymerization such as polyethylene (PE), polypropylene (PP), polyvinyl chloride (PVC), polymethyl methacrylate, polyacrylonitrile, polyvinyl acetate.
vi. Define the terms :
1) Monomer : Chemically polymers are complex, giant macromolecules made from the repeating units which are derived from small molecules called ‘monomers’
2) Vulcanisation: The process by which a network of cross links is introduced into an elastomers is called vulcanization.
3) Synthetic fibres: Synthetic fibers are fibers made by humans through chemical synthesis, as opposed to natural fibers that are directly derived from living organisms.
vii. What type of intermolecular force
leads to high density polymer ?
Answer : HDPE mostly features a low degree of branching where the linear molecules or the polymer chains are packed together tightly. The presence of a strong intermolecular force results in a dense, highly crystalline material.
viii. Give one example each of copolymer and homopolymer.
Answer : In other words, the repeating units of homopolymers are derived only from one monomer. For example, polythene is a homopolymer of ethane. The polymers whose repeating units are derived from two types of monomers are known as copolymers. For example, Buna−S is a copolymer of 1, 3-butadiene and styrene.
ix. Identify Thermoplastic and Thermosetting Plastics from the
following —–
Answer :
1. PET- thermoplastic
2. Urea formaldehyde resin- thermosetting
3. Polythene-thermoplastic
4. Phenol formaldehyde – thermosetting
3. Answer the following.
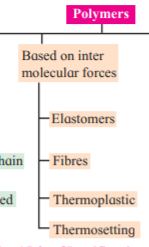
i. Write the names of classes of polymers
formed according to intermolecular
forces and describe briefly their
structural characteristics.
Answer :
ii. Write reactions of formation of :
a. Nylon 6
Answer :
b. Terylene
Answer :