Class 12 chemistry chapter 16 Green Chemistry And Nanochemistry
Maharashtra state Chemistry Textbook Solutions for Class 12 are very important and crusial that helps the students in understanding the complex topics and helps them in the preparation of class 12 board examination as well as verious compititive entrance examinations also. Studying the answers to the questions in the Chemistry textbook will check your understanding of a particular topic and helps you determine your strengths and weaknesses.
Class 12 chemistry textbook Solutions for Class 12, Chemistry Chapter 16 Green Chemistry And Nanochemistry maharashtra state board are provided here with simple step-by-step detailed explanations. These solutions for Green Chemistry And Nanochemistry are very popular among Class 12 students for chemistry chapter 16 Green Chemistry And Nanochemistry Solutions come handy for quickly completing your homework and preparing for exams. All questions and answers from the chemistry textbook Solutions Book of Class 12 chemistry Chapter 16 are provided here for you for free. You will also love the experience on ybstudy class 12 Solutions. All chemistry textbook Solutions. Solutions for class 12, These chemistry textbook solutions are prepared by Chemistry experts and are 100% accurate.
1. Choose the most correct option.
i. The development that meets the needs
of present without compromising the
ability of future generations to meet
their own need is known as
a. Continuous development
b. Sustainable development
c. True development
d. Irrational development
ii. Which of the following is ϒ-isomer of BHC?
a. DDT
b. lindane
c. Chloroform
d. Chlorobenzene
iii. The prefix ‘nano’ comes from
a. French word meaning billion
b. Greek word meaning dwarf
c. Spanish word meaning particle
d. Latin word meaning invisible
iv. Which of the following information is given by FTIR technique ?
a. Absorption of functional groups
b. Particle size
c. Confirmation of formation of
nanoparticles
d. Crystal structure
v. The concept of green chemistry was
coined by
a. Born Haber
b. Nario Taniguchi
c. Richard Feynman
d. Paul T. Anastas
2. Answer the following
i. Write the formula to calculate % atom
economy.
Answer : % atom economy =
Formula weight of the desired product
/ sum of formula weight of all the reactants used in the reaction × 100
ii. Name the ϒ-isomer of BHC.
Answer : Lindane, also known as gamma-hexachlorocyclohexane (γ-HCH), gammaxene, Gammallin and sometimes incorrectly called benzene hexachloride (BHC), is an organochlorine chemical and an isomer of hexachlorocyclohexane that has been used both as an agricultural insecticide and as a pharmaceutical treatment for lice and scabies.
iii. Ridhima wants to detect structure of surface of materials. Name the technique she has to use.
Answer : Various techniques such as optical microscopy, scanning electron microscopy, tunneling electron microscopy, atomic force microscopy, X-ray photoelectron spectroscopy, Fourier transform infrared spectroscopy, X-ray diffraction, 3D profilometry, and Raman spectroscopy are used to define surface structures, frequency
iv. Which nanomaterial is used for tyres
of car to increase the life of tyres ?
Answer : Small particles of carbon black (including nanoparticles) have long been mixed with rubber to improve the wear and strength of tires. Many leading tire manufacturers are now developing engineered nanoparticles to further extend tire life.
v. Name the scientist who discovered
scanning tunneling microscope (STM)
in 1980.
Answer : Binnig, Rohrer, and colleagues at the IBM laboratory in Zürich pioneered the development of scanning tunneling microscopy (STM) in the early 1980s, Binnig and Rohrer being awarded a Nobel prize in 1986.
vi. 1 nm = …..m ?
Answer : 1 nm = 1 × 10-9 metre
3. Answer the following
i. Define
(i) Green chemistry : Green Chemistry is the use of chemistry for pollution prevention by environmentally conscious design of chemical products and processes that reduce or eliminate the use or generation of hazardous substances.
(ii) sustainable development : Sustainable development is development that meets the needs of the present, without compromising the ability of future generations to meet their own need.
ii. Explain the role of green chemistry.
Answer : To promote innovative chemical
technologies that reduce or eliminate the use or generation of hazardous substances.
The green chemistry helps to reduce
capital expenditure, to prevent pollution.
Green chemistry incorporates pollution
prevention practices in the manufacture of
chemicals and promotes pollution prevention and industrial ecology.
Green chemistry helps to protect the
presence of ozone in the stratosphere essential for the survival of life on the earth. Green chemistry is useful to control green house effect (Global warming)
iii. Give the full form (long form) of the
names for following instruments.
a. XRD = X-ray Powder Diffraction
b. TEM. = Transmission electron microscopy
c. STM
= scanning tunneling microscope
d. FTIR = Fourier-transform infrared spectroscopy
e. SEM = scanning electron microscope.
iv. Define the following terms :
a. Nanoscience
Answer: Nanoscience is the study of phenomena and manipulation of materials at atomic, molecular and macromolecular scales where properties differ significantly from those at a larger scale.
b. Nanotechnology
Answer : Nanotechnology is the design, characterization, production and application of structures, device and system by controlling shape and size at nanometer scale.
c. Nanomaterial
Answer : The nanomaterial is a material having structural components with atleast one dimension in the nanometer scale that is 1-100 nm.
d. Nanochemistry
Answer : It is the
combination of chemistry and nanoscience.
v. How nanotechnology plays an important role in water purification
techniques?
Answer : Water contains waterborne pathogens like viruses, bacteria. 1.1 billion people are without access to an improved water supply. The provision of safe drinking water is currently
high priority. Recently, cost effective filter
materials coated with silver nanoparticles
(AgNps) is an alternative technology. (For
example : water purifier) Silver nanoparticles act as highly effective bacterial disinfectant,
remove E.Coli from water.
vi. Which nanomaterial is used in sunscreen lotion ? Write its use.
Answer : Many mineral sunscreens use nano sized zinc oxide because it is less whitening and therefore more aesthetically appealing than larger particle zinc oxide.
vii. How will you illustrate the use of
safer solvent and auxiliaries ?
Answer : The principle states that the use of solvents and other auxiliaries should “be made unnecessary wherever possible and innocuous when used”. Because they do matter it is important that safer solvents are used.
viii. Define catalyst. Give two examples.
Answer : A catalyst is a substance that speeds up a chemical reaction. Common types of catalysts include enzymes, acid-base catalysts, and heterogeneous (or surface) catalysts.
4. Answer the following
i. Explain any three principles of green
chemistry.
Answer :
1. Prevention of waste or by products :
To give priority for the prevention of
waste rather than cleaning up and treating
waste after it has been created.
2. Atom economy : Atom economy is a
measure of the amount of atoms from the
starting materials that are present in the useful
products at the end of chemical process.
3. Less hazardous chemical synthesis :
Designed chemical reactions and
synthesis routes should be as safe as possible.
So that we can avoid formation of hazardous
waste from chemical processes.
4. Desigining Safer Chemicals : This principle
is quite similar to the previous one. To develop products that are less toxic or which require
less toxic raw materials.
ii. Explain atom economy with suitable
example.
Answer : Atom economy is a
measure of the amount of atoms from the
starting materials that are present in the useful products at the end of chemical process. Good atom economy means most of the atoms of the reactants are incorporated in the desired
products and only small amounts of unwanted byproducts are formed and hence lesser problems of waste disposal.
For example : conversion of Butan-1-ol to 1 – bromobutane
CH3- CH2-CH2-CH2-OH + NaBr + H2
SO4
——–> CH3-CH2-CH2-CH2-Br + NaHSO4
+ H2O
% atom economy =
mass of (4C + 9H + 1Br) atoms
/ mass of (4C + 12H + 5O + 1Br + 1Na + 1S)atoms × 100
= 137 u / 275 u × 100
= 49.81 %
iii. How will you illustrate the principle,
minimization of steps ?
Answer : A commonly used technique in
organic synthesis is the use of protecting or
blocking group. Unnecessary derivatization
(for example installation / removal of use of protecting groups) should be minimized or
avoided if possible, because such steps require additional reagents and can generate waste.
Illustration : In organic synthesis, we need
very often protection of some functional
groups. Finally, we again need their
deprotection. It is explained in the following example of synthesis of m-hydroxybenzoic acid from m-hydroxy benzaldehyde. Obviously, in such cases, atom economy is also less. The green chemistry principle aims to develop the methodology where unnecessary steps should be avoided, if practicable biocatalytic reactions very often
need no protection of selective group.
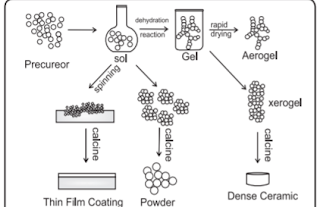
iv. What do you mean by sol and gel? Describe the sol-gel method of
preparation for nanoparticles.
Answer :
Sol-gel process : Sols are
dispersions of colloidal particles in a liquid.
Colloids are solid particles with diameters of 1-100nm. A gel is interconnected rigid network. A sol-gel process is based on inorganic polymerization reactions. It is generally carried out at room temperature and includes four steps : hydrolysis, polycondensation, drying and thermal decomposition. This method is
widely employed to prepare oxide materials.
1. Formation of different stable solution of the alkoxide or solvated metal precursor.
2. Gelation resulting from the formation of an oxide or alcohol-bridged network. (gel) by a polycondensation reaction.
3. Aging of the gel means during that period gel transforms into a solid mass.
4. Drying of the gel : In this step, water and
other volatile liquids are removed from the
gel network.
5. Dehydration : The material is heated at
temperatures upto 800°C.
v. Which flower is an example of self cleaning ?
Answer : lotus plant (genus Nelumbo) really does have self-cleaning leaves. Or, more accurately, the lotus has leaves that are so water-repellant that water beads on the surface and, as it rolls off, picks up any dirt or debris – all done without a chemical coating.