Class 7 Science Chapter 3 Properties of Natural Resource Textbook Solutions
Maharashtra State Board Class 7 Science Solutions Chapter 3 Properties of Natural Resources:
Important points to remember :
- The molecules of the gases in the air are in constant motion. When these molecules strike a body, they create pressure on that body. This is the pressure of air that we call ‘atmospheric pressure.
- Under ordinary conditions, atmospheric pressure at sea level is about 1,01,400 Newtons per square meter.
- In 1726, Swedish scientist Daniel Bernoulli put forth the important principle that the pressure of air decreases when its velocity increases while the pressure of air increases when its velocity decreases.
- Air is a mixture of very fine particles of some gases, dust, smoke, and moisture.
- When rays of light fall on these minute particles, the particles spread the light in all directions. This natural phenomenon is called the scattering of light.
- Air is useful as a medium for the transmission of sound.
- water expands when the temperature falls below 40 C. This is called the anomalous behavior of water.
- The Danish Scientist Sorensen put forth the concept of pH, based on the concentration of hydrogen ions. World Soil Day: 5th December
General Science Solutions for Class 7 Science Chapter 3 – Properties Of Natural Resources:
(Temperature, volume, mass, density, humidity, acidic, weight, neutral, shape.)
a) The capacity of air to hold moisture depends upon the temperature of the air.
b) Water does not have a shape but has definite mass and volume.
c) While freezing, the density of water is lowered.
d) Neutral soil has a pH of 7.
Question 2: Why is it said that –
a) Air is a homogeneous mixture of various gases.
Answer: Air is a homogeneous mixture of gases as it is primarily made up of nitrogen and oxygen. Its elements are not readily separated or distinguished from one another.
b) Water is a universal solvent
Answer: Water is a very good solvent as it can dissolve almost every substance in it, therefore water is known as the “universal solvent”.
c) There is no alternative to water for cleaning purposes.
Answer: It is said that there is no alternative to water for cleaning purposes. Water is provided by nature. Water is available in abundance. So as there is more water on Earth we use water for cleaning purposes. We do not use other substances because some substances are very expensive and some substances are very scarce.
Question 3: What will happen if…
a) The amount of water vapor in the air increases
Answer: If the amount of water vapor in the air increases, the relative humidity increases, and if the amount of water vapor in the air decreases, the relative humidity decreases.
b) Only one crop is grown repeatedly.
Answer: Is called monoculture. The practice of growing only one type of crop on the same field is correctly referred to as a monoculture. Monoculture refers to the growing of the same kind of crops on the same field repeatedly.
Question 4: With whom should I pair up?
Group ‘A’. Group ‘B’
1) Air. a) Excretion
2) Water. b) Scattering of light
3) Soil. c) Plasticity
Answer :
1) Air. – b) Scattering of light
2) Water. – a) Excretion
3) Soil. – c) Plasticity
Question 5: State whether the following statements are true or false.
a) Sandy soil has a low capacity for holding water.- False
b) Sea water is a bad conductor of electricity.- False
c) The substance in which a solute dissolves is called a solvent.- True
d) The pressure exerted by air is called atmospheric pressure.- True
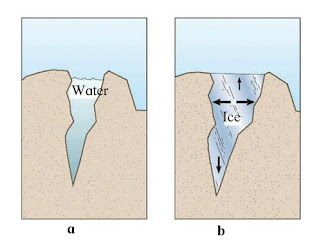
Question 6: Explain the picture in your own words.
Answer: Image ‘a’ represents a crack that is filled with water whereas image ‘b’ represents a crack that has ice. It is evident from both the images that in image ‘b’, the width of the crack increases as the water in the crevice freezes. This is one of the properties of water due to which it expands upon freezing. This expansion in water after freezing results in the widening of the crack in image ‘b’.
Question 7: Write answers to the following questions in your own words.
a) How is lightly scattered by the air?
Answer:
- The phenomenon occurs when a ‘parallel beam of light passes through a particle present in the air, the particle scatters the light and illuminates it in all directions apart from its incident direction.
- The ‘atmospheric air molecules are smaller than the visible light wavelength range.
- The lower wavelength light like blue is ‘scattered more than the higher ones like red and this is the reason the sky appears blue.
- The sunlight falling on a particle in the air is scattered according to its wavelength and not as white light.
- This scattering of light by air is also termed Rayleigh’s Effect.
b) Explain the various properties of water.
Answer: The main properties of water are its polarity, cohesion, adhesion, surface tension, high specific heat, and evaporative cooling.
- Polarity: A water molecule is slightly charged on both ends. This is because oxygen is more electronegative than hydrogen.
- Adhesion: Similar to cohesion, adhesion is when the hydrogen bonds in water allow for the water molecules to be held to another substance.
- Heat: Specific heat is the amount of heat absorbed or lost for 1g to change 1ºC, which in the case of water, is pretty high. This allows for evaporative cooling to occur, which is when heat energy is transferred to water molecules, and evaporating water removes a lot of heat energy from an organism
- Cohesion: Hydrogen bonds hold water molecules together, as seen in the picture above. Cohesion creates surface tension which is why if you fill a spoon with water, drop by drop, the water volume will actually be bigger than the spoon’s surface before the water falls off.
- Other important characteristics of water involve it being a universal solvent, along with its unusual density. Water, unlike any other solid-liquid, is denser in its liquid form than as a solid, which is why ice floats, and this allows for entire habitats to exist underneath layers of ice floating on oceans. Its neutral pH (7) is also a relevant characteristic.
c) Why is the density of seawater more than that of rainwater?
Answer:
- Sea water has more density than freshwater or rainwater.
- It is because there are salt ions present in the seawater which makes the seawater heavier, it means the mass of the water increases due to the presence of salt ions.
- The density of seawater ranges between 1020 and 1029 kg/m³. As we know that the mass is directly proportional to the density,
- Density = mass/ volume. that’s why the density of seawater is more than that of fresh or rainwater.
d) What is the importance of good soil structure?
Answer:
- Soil structure is the arrangement of soil particles (sand, silt, clay, and organic matter) into granules, crumbs, or blocks.
- It is the shape that the soil takes based on its physical, chemical, and biological properties.
- Soil structure is often confused with soil texture, both of which affect the soil’s drainage and aeration capabilities.
- A good soil structure is important to allow air and water into the soil which are vital for healthy plant growth.
- It will improve drainage and reduce soil erosion caused by excess surface run-off.
- Without structure, soils will suffer from anaerobic, water logging, and nutrient lock-up, and, ultimately, plants will die!
e) What are the various uses of soil?
Answer: Uses of Soil:
- Plant conservation: To help plants grow.
- Water conservation: Soil holds water. As a result, using bunds and lakes, we can get water for use throughout the year.
- Plasticity: Soil can be given any required shape. This property of soil is called plasticity. Because of its plasticity, we can use it to make articles of a variety of shapes. These articles can be baked to make them hard. Water storage earthen pots, earthen lamps, idols, bricks, etc. are articles made from soil.
- Soil is used in agriculture, where it serves as the primary nutrient base for plants. Soil resources are critical to the environment, as well as to food and fiber production.
- Soil is used in constructions and arts. Soil material is a critical component in the mining and construction industries.
- Soil serves as a foundation for most construction projects. Soil plays an important role in filtrating and purifying water.
- After coming down as precipitation, much of the rainwater is percolated through the many horizons of a soil profile and renamed as groundwater.
- Waste management often has a soil component. Landfills use soil for daily cover.
- Septic drain fields treat septic tank effluent using aerobic soil processes.
- Organic soils, especially peat, serve as a significant fuel resource.
f) What is the need and importance of soil testing from the point of view of farmers?
Answer:
- The proportions of the various ingredients in the soil can be determined by ‘soil testing.
- During soil testing, the soil is examined for color, texture, and the proportion of organic matter in it.
- Soil is tested to find out if there is a deficiency of any ingredients.
- The soil test analyses the levels of macronutrients, micronutrients, and pH present in the soil and balances them with the crop’s nutrient demands.
- It is also vital to observe the levels of degradation of land.
- Soil testing analyses are important for farmers to better understand their soil types and deficiencies of nutrients and it helps to minimize the quantity and different types of fertilizers, which becomes a cost-benefit to peasants.
g) What is the importance of air in the transmission of sound?
Answer: The air is the medium that is necessary for the transmission of sound. The sound needs a medium to propagate and air provides that medium. Sound waves are created with help of the disturbances in the medium.
h) Why should a glass bottle completely filled with water never be kept in a freezer?
Answer: The expansion of water takes place as it is cooled from 4°C to 0°C. This behavior is unusual because most substances contract when they are cooled. Due to this expansion, a glass bottle completely filled with water and tightly closed at room temperature is likely to burst when kept in a freezer of a refrigerator.
# let’s recall
1. What are the gases present in the air?
Answer: By volume, dry air contains 78.09% nitrogen, 20.95% oxygen, 0.93% argon, 0.04% carbon dioxide, and small amounts of other gases. Air also contains a variable amount of water vapor, on average around 1% at sea level, and 0.4% over the entire atmosphere.
2. Why is air called a homogeneous mixture? What are the uses of the various gases in the air?
Answer: Air is said to be a Homogeneous mixture of different gases because it contains different gases like Nitrogen, oxygen, carbon dioxide, argon, etc. This is said to be a homogeneous mixture because the constituent cannot be distinguished, unlike a heterogeneous mixture.
# Use your brain
1. What is the effect of increased temperature on the pressure of air?
Answer: Gay-Lussac’s Law is one part of the ideal gas law and so explains how gases change when the volume is held constant. As the temperature increases, the molecules in the gas move faster, impacting the gas container more frequently and exerting a greater force. This increases the pressure.
2. What are the states in which water is found?
Answer: Water occurs in a liquid state under ordinary conditions. Water is a fluid substance. Water does not have its own shape, but has a volume. It can pass through small holes or seep through very small gaps or cracks.
Tags: natural resources standard 7, 7 standard science question answer, general science solution for class 7, 7th standard general science digest, properties of natural resources std 7, materials we use class 7, state board class 7 science notes, natural resources and their conservation class 7