Science Textbook Solutions for Class 8 Science Chapter 5 – Inside the Atom
Important points to remember :
- The concept of atom is more than 2500 years old. However, it was forgotten in the course of time.
- In the modern times, scientists on the basis of experiments explained the nature of atom as well as the internal structure of atom.
- It started with Dalton’s atomic theory. Indian philosopher Kanad (6th century B.C.) stated that there is a limit to divide matter into small particles.
- The indivisible particles that constitute matter were named by Kanad Muni as ‘Paramanu’ (meaning the smallest particles). He also stated that ‘Paramanu’ is indistructible. ·
- Greek philosopher Democritus (5th century B.C.) stated that matter is made of small particles and these cannot be divide.
- The smallest particle of matter was name by Democritus as ‘Atom’. (In Greek language ‘Atomos’ means the one which cannot be cut.
- British scientist John Dalton put forth in 1803 A.D. his celebrated ‘Atomic Theory’. According to this theory matter is made of atoms and atoms are indivisible and indestructible.
Question 1: Answer the following
A. Whati is the difference in the atomic models of Thomson and Rutherford ?
Answer : Difference between Thomson and Rutherford Theory are as follows :
- Thomson Theory States that electron are embedded in a positively charged solid material which is spherical in shape
- Rutherford theory : States that an atom is composed of an atomic nucleus around which electrons are revolving in an orbit
- Thomson theory Does not give any detail about the atomic nucleus
- Rutherford theory Explains about the atomic nucleus
- Thomson theory States that electrons are uniformally distributed in an atom
- Rutherford theory States that electrons are located around a central solid material.
B. What is meant by valency of an element ? what is the relationship between the number of valence electron and valency ?
Answer : Valency : It is the number of electrons of an atom of the element uses to combine with atoms of other elements.
Valency of an atom is determined by its electronic configuration.
Number of valence electron : It is defined as the number of electrons present in the outermost shell of an atom.
It is not necessary that all the valence electrons take part in bonding.
For example: Na(11) = 2,8,1 So, its number of valence electron is 1
Cl(17) = 2,8,7 So its number of valence electron is 7
It can be seen that valency of an element is related to number of valence electrons in that atom.
C. What is meant by atomic mass number ? Explain how the atomic number and mass number of carbon are 6 and 12 respectively.
Answer : Atomic Mass Number : It is the sum of total number of protons and neutrons present in a nucleus.
Atomic mass number = number of protons + number of neutrons.
Atomic number = number of protons = number of electrons. It is denoted by Z.
Mass number = number of protons + number of neutrons. It is denoted by A.
For example : Carbon atom its, Number of proton = 6
Number of neutrons = 6
Number of electron = 6
Atomic number(Z) = number of protons = number of electrons = 6.
Mass number(A) = number of proton + number of neutrons = 6 + 6 =12.
D. what is meant by subatomic particle ? give brief information of three subatomic particles with refrence to electrical charge, mass and location.
Answer : Subatomic particles : A subatomic particle is a structural and functional unit of the matter. That means all the matters are made up of these fundamental particles. According to modern atomic theory, an atom has a nucleus, which is present in its center or core. These nucleus contain subatomic particles like protons and neutrons.
Difference between subatomic particles are as follows :
Electron
1. Electrons are present outside the nucleus of an atom.
2. Electrons are negatively charged that is (1.6 × 10−191.6 × 10-19coulomb).
3. The mass of an electron is considered to negligible. It is 1800 times less than that of a hydrogen
4.Relative mass = 1/ 1840 times hydrogen
5. It revolves around the nucleus in the discrete orbit.
Proton
1. Protons are present in the nucleus of an atom.
2. Protons are positively charged that is (1.6 × 10−191.6 × 10-19coulomb).
3. The mass of a proton is approximately 1u(1Dalton) that is (1u = 1.66 × 10−27 g1u = 1.66 × 10-27 g).
4. They are closely bound in the nucleus.
Neutron
1. Neutrons are present in the nucleus of an atom.
2. Neutrons are neutral.There is no charge.
3. The mass of a neutron is nearly equal to the mass of a proton that is 1u(1Dalton) that is (1u = 1.66 × 10−27 g1u = 1.66 × 10-27 g).
4. They are closely bound in the nucleus.
Question 2: Give secientific reasons.
A. All the mass of an atom is concenrated in the nucleus.
Answer : All the mass of an atom is concentrated in the nucleus because atom contains three subatomic particles like electron, proton and neutron.Out of which, nucleus present at the centre of an atom contains two subatomic particles that’s protons and neutrons and the mass of nucleus is the sum of mass of protons and neutrons located at the centre of an atom.
B. Atom is electrically neutral.
Answer : Atom is electrically neutral because in an atom electrons and protons carry charges and each atom has equal numbers of protons (positively charged) and electrons (negatively charged).
C. Atomic mass number is a whole number.
Answer : Atomic mass number is a whole number because it is the sum of numbers of protons and numbers of neutrons present in an atom.Which is present in the form of integers.
D. Atoms are stable though negatively charged electron are revolving within it.
Answer : Atoms are stables though negatively charged electrons are revolving within it because each atom contains equal numbers of protons and electrons. So, charge on negatively charged electrons are balanced by charge on positively charged proton. Therefore, atom is electrically neutral and stable.
Question 3: Define the following forms
a. Atom
Answer : An atom is defined as the structural and functional unit of a matter. atoms are the smallest things in the universe and could not be divided. atoms are made up of three subatomic particles like protons, neutrons and electrons.
b. Isotopes
Answer : Isotopes are atoms that have same atomic number but different mass number. Isotopes have same numbers of protons but different numbers of neutrons.
c. Atomic number
Answer : The number of protons in the nucleus of an atom, which is characteristic of a chemical element and determines its place in the periodic table. Atomic number is also equal to numbers of electrons in an atom.
d. Atomic Mass Number
Answer : It is the sum of total number of protons and neutrons present in a nucleus.
atomic mass number = number of protons + number of neutrons.
e. Moderator in nuclear reactor:
Answer : Moderator of a nuclear reactor is a substance that slows down the speed of neutrons. In traditional nuclear reactors, the moderator is the same thing as that of coolant like water.
Question 4: Draw a neat lablled diagram
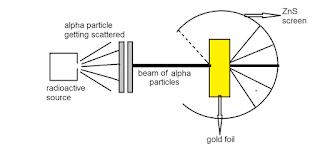
A. Ruthrford’s scattering experiment
B. Thomson’s atomic model
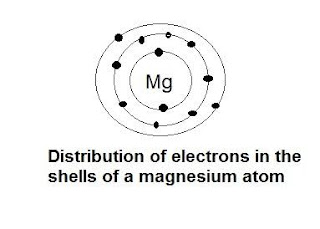
C. Diagramatic sketch of electronic configurations of magnesium (Atomic number 12)
D. Diagramatic sketeh of electronic configuration of Argon (Atomic number 18)
Question 5: Fill in the blanks.
a. Electron, proton, neutron are the types of subatomic particles in an atom.
b. An electron carries a negative charge.
c. The electron shell K is nearest to the nucleus.
d. The electronic configuration magnesium is 2, 8, 2. From this it is understood that the valence shell of magnesium is M shell.
e. The valency of hydrogen is ‘one as per the molecular formula H2O . Therefore valency of ‘Fe’ turns out to be three as per the formula Fe2O3.
Question 6: Match the pairs.
Group ‘A’. Group ‘B’
a. Proton 1. Negatively charged
b.Electron 2. Neutral
c.Neutron 3.Positively charged
Answer :
a.Proton 1.Positively charged
b.Electron. 2.Negatively charged
c.Neutron 3.Neutral
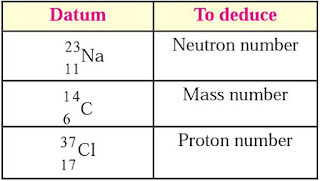
Question 7: Deducd from the datum provided.
Answer :
# use your brain power
1. Which discovery did point out that an atom has internal structure?
Answer : Rutherford’s Gold Foil Experiment proved the existance of a small massive center to atoms, which would later be known as the nucleus of an atom. Ernest Rutherford, Hans Geiger and Ernest Marsden carried out their Gold Foil Experiment to observe the effect of alpha particles on matter.
2. What is the difference between the solid atom in Dalton’s atomic theory and Thomson’s atomic model?
Answer : Dalton’ atomic theory tells about that electrons move in shells or orbit. As well as Thomson said that there are positive charges in the centre and it is the nucleus. Normally Dalton’s theory stated that atoms were indivisible, in the energy Democritus’ model of the atom was simply a round, solid ball.
3. Explain the difference between the distribution of positive charge in Thomson’s atomic model and Rutherford’s atomic model.
Answer : The positive charge has to be concentrated in a very small volume to deflect the positively charged α-particles. Rutherford’s calculations show that the volume of the nucleus is very small compared to the total volume of the atom.
4. What is the point difference between the place of electron in the atomic models of Thomson and Rutherford?
Answer : He was able to reach to dense nucleus in between but at the same time could not explain the stability. 2. he explained electrons were embedded uniformly in a positively charged matrix.
5. What is the thing which is present in Rutherford’s atomic model and not present in Dalton’s and Thomson’s atomic models?
Answer : Nucleus is the thing which is present in Rutherford’s atomic model and not present in Dalton’s and Thomson’s atomic models. In 1911, Rutherford coined the planetary model of atom with the help of his gold-foil experiment. In this model, he bombarded alpha particles from radioactive elements on the shining screen.
#Use your brain power
1. How many types of subatomic particles are found in atom?
Answer : The three main subatomic particles that form an atom are protons, neutrons, and electrons. The center of the atom is called the nucleus. First, let’s learn a bit about protons and neutrons, and then we will talk about electrons a little later. Protons and neutrons make up the nucleus of an atom.
2. Which subatomic particles are electrically charged?
Answer : Protons, neutrons, and electrons are the three main subatomic particles found in an atom. Protons have a positive (+) charge. An easy way to remember this is to remember that both proton and positive start with the letter “P.” Neutrons have no electrical charge.
3. Which subatomic particles are present in the nucleus?
Answer : The nucleus contains two types of subatomic particles, protons and neutrons. The protons have a positive electrical charge and the neutrons have no electrical charge. A third type of subatomic particle, electrons, move around the nucleus. The electrons have a negative electrical charge.
4. Where are electrons revolving around the nucleus placed?
Answer: The atom’s center, or nucleus, is positively charged and the electrons that whirl around this nucleus are negatively charged, so they attract each other.
Tags : structure of atom class 8 pdf, structure of atom – class 10, structure of atom class 8 ppt, structure of atom class 7 cbse, structure of atom class 9, atomic structure notes, atom is electrically neutral