MCQs on haloalkanes & halorenes for NEET | pdf
MCQs on haloalkanes & halorenes for NEET | pdf
MCQs on haloalkanes & halorenes for NEET is a very important chapter for NEET Aspirants. NEET exam is important in your life, because your future career depends upon your score in NEET exam MCQs on haloalkanes & halorenes for NEET test your knowledge, intelligence, memory and quick response. Your speed and accuracy is the essence of this NEET MCQ. For this you have to cultivate a different frame of mind. For this first solve the different MCQs on haloalkanes & halorenes for NEET | pdf given in this page and then try to complete each Chapter of chemistry NEET MCQs given on ybstudy.com.
MCQs on haloalkanes & halorenes for NEET With Our MCQs on haloalkanes & halorenes for NEET|pdf Find out where you stand, your strong points and weak points and try to take corrective steps immediately. In this process your subconscious mind will be thinking about the correct answers of MCQs on haloalkanes & halorenes for NEET for those questions and in the second round, you will be getting most of the answers. Remember before starting to solve our MCQs on haloalkanes & halorenes for NEET|pdf online test all the important notes and the meaning of all definitions should be understood
MCQs on haloalkanes & halorenes for NEET
1) Ethyl bromide when reacted with silver salt of ethanoic acid it gives
a) diethyl ether
b) ethyl alcohol
c) ethyl ethanoate
d) ethanoic anhydride
Answer : c
2) Which of the following organic compound contain co-ordinate bond?
a) alkyl amine
b)alkyl cyanide
c) acid amide
d) alkyl isocyanide
Answer : d
3) An alcohol which take about 5-minutes to develope turbidity with Lucas reagent is __________
a) n-butyl alcohol
b) sec. butyl alcohol
c) iso butyl alcohol
d) tert bubyl alcohol
Answer : b
3) When tert butyl alcohol is refluxed with NaBr and conc. H2SO4, it predominately gives__________
a) tert. butyl bromide
b) 2- methyl propene
b) tert. butyl hydrogen sulphate
d) 2-methyl propane
Answer : b
4) The best of alkyl iodide is obtained when alcohol is heated with_________
a) Nal + 95 % H3PO4
b) Nal + conc. H2SO4
c) Conc. H3PO4
d) Conc. HI
Answer : a
5) Which of the following phosphorus halides does not exist?
a) PCl3
b) PBr3
c) PCl5
d) PBr5
Answer : d
6) Alkyl is conviniently prepared from alcohol by using__________
a) phosphorus pentachloride
b) sulphonyl chloride
c) conc. hydrogen chloride
d) phosphorus trichloride
Answer : b
7) When 3-methylbutan-2-ol is heated with sodium bromide and dil. H,sO,. The major product formed Is
a) 2-bromo-3-methylbutane
b) 2-bromo-2-methyl butane
c) 3-methyl but-1-ene
d) 2-methylbut-2-ene
Answer : b
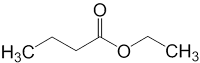
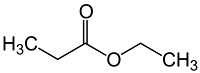
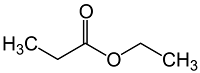
8) which of the following is ethyl propionate?
a)
b)
C)
d)
Answer : d
9) Iso butyl halide is an example of______ alkyl halide
a) 1°alkyl halide
b) 2° alkyl halide
c) 3° alkyl halide
d) 4° alkyl halide
Answer : a
10) Which of the following is primary halide?
a) iso-propyl halide
b) sec-butyl halide
c) tert-butyl halide
d) neo-hexyl chloride
Answer : d
11) In tertiary butyl bromide the tertiary carbon atom is bonded with________
a) no hydrogen atom
b) two hydrogen atoms
c) three hydrogen atoms
d) one hydrogen atom
Answer : a
12) Mono halogen derivatives of alkanes are known as________
a) alkynyl halides
b) alkyl halide
c) aryl halide
d) alkeny halide
Answer : b
13) Iso-butyl chloride and n-butyl chloride are________
a) position isomers
b)chain isomers
c) functional isomers
d) metamers
Answer : c
14) Grignard’s reagent is prepared by the reaction between_______
a) zinc and alkyl halide
b) magnesium and alkane
c) magnesium and alkyl halide
d) magnesium and aromatic hydrocarbon
Answer : c
15) When 1-chloropropane is treated with alc. potash gives propene. The reaction is__________
a) dehydration
b) substitution
c) addition
d) elimination
Answer : d
16) Dehydrochlorination of (CH3)2 C HCHCICH3 gives_________
a) (CH3)2 C = CHCH3
b) (CH3)2 CH-CH = CH2
c) Both ‘a’ and ‘b’
d) (CH3)2 CHCHOHCH3
Answer : a
17) Among primary secondary and tertiary alkyl halides the order of reactivity towards cyanolysis is (-R remains same)
a) 1°>2°>3°
b) 3°>2°> 1°
c) 2°> 1°>3°
d) all have same reactivity
Answer : a
18) Butane nitrile is prepared from
a) butyl chloride and KCN
b) butyl alcohol and KCN
c) propyl alcohol and KCN
d) propyl chloride and KCN
Answer : d
19) An alkyl halide with an active metal containing 11electrons to give saturated hydrocarbon. The reaction is known as_______
a) Williamson’s synthesis
b) Hoffmann’s method
c) Wurtz fitting reaction
d) Wurtz coupling reaction
Answer : d
20) Grignard reagent undergo __________
a) nucleophilic addition
b) electrophilic substitution
C) nucleophilic substitution
d) electrophilic addition
Answer : a
21) When isomers have the same structural formula but differ in the relative arrangement of their atoms or groups, then they are called_________
a) stereoisomers
b) optical isomers
c) mesomers
d) geometrical mesomers
Answer : a
22) A racemic mixture consist of
a) different amounts of enantiomers
b) equal amounts of enantiomers
c) only dextro forms
d) only laevo forms
Answer : b
23) An optically active compound is_____
a) 1-bromobutane
b) β-bromobutyric acid
c) 2-bromo-2-methylpropane
d) 1-bromo-2-methylpropane
Answer : b
24) If a compound has ‘n’ chiral centres, then possible number of stereoisomers (or optical isomers) is_____
a) 2n
b) 2√n
c) √2n
d) 2n-1
Answer : a
25) Some statements are given about racemate
1. It may rotate plane of plane polarised light towards both the side
2. It is a mixture ol wo optical isomers
in equimolar proportions
3. It is mixture of two optical isomers in equal proportions by weight
4. It is optically inactive by exiemal compensation.
Among the above, the correct statement(S) is/are________
a) only 4
b) only 2
c) onlyI and 2
d) only 2 and 4
Answer : d
26) Which of the following is/are not optically active compound(s)?
a) CH3CHBrC2H5
b) CH3CHOHC2H5
c) CH3CHCNC2H5
d) CH3-C-Br2C2H5
Answer : d
27) How many optically active forms are possible for a compound of formula_________
HOCH2CHOHCHOHCHOHCH2OH
a) 8
b) 2
c) 3
d) 4
Answer : a
28) Among the following which one has a chiral structure?
a) 1-chloro-2,2-dimethyl propane
b) 2-methyl-2-chloro propane
c) 2-chloro pentane
d) 3-chloro pentane
Answer : c
29) If dextro-rotatory compound undergoes SN2 reaction the product will be________
a) dextro-rotatory
b) laevo-rotatory
C) racemic mixture
d) parialy optically active
Answer : b
30) Number of asymmetric carbon atoms in 2, 2- dibromobutane will be________
a) 4
b) 2
c) 1
d) 0
Answer : 0
31) lodobenzene can be converted into diphenyl by_______
a) Wartz reaction
b) Ullman reaction
c) Wurtz Fitting reaction
d) Frankland reaction
Answer : b
32) Which of the tollowing represents Freon?
a) acetylene tetrachloride
b) trichloroethylene
c) dichlorodifluoromethane
d) ethylene dichloride
Answer : c
33) How many position isomers are possible for dichlorobenzene?
a) 3
b) 2
c) 4
d) 5
Answer : d
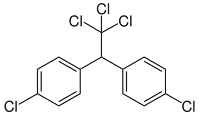
34)
The above structural formula refers to
a) BHC
b) DDT
c) DNA
d) RNA
Answer : b
35) On sulphonation of C6H5CI
a) m-Chlorobenzenesulphonic acid is formed
b) Benzenesulphonic acid is formed
C) o-Chlorobenzenesulphonic acid is formed
d) O-and p-Chlorobenzenesulphonic acid is formed
Answer : d
36) Anisole is the product when chlorobenzene is treated with ______
a) Cupious cynid
b) Ammona
c) Sodium methoxde
d) Sodiunm hydroxide
Answer : c
37) Aromatic ketones can be obtained from chlorobenzen by ________
a) Friedal-crafts acetylation
b) nitration
c) Friedal-craft alkylation
d) none of these.
Answer : a
38) In the nitration ot coroeee, the major product is________
a) o-nitrochlorobenezene
b) p-nitrochlorobenezene
c) 3- nitrochlorobenezene
d) 3,5 dinitrochlorobenzene.
Answer : b
39) The maximum number of 1somers possible with the aromatic compound, with molecular formula C7H7X is________
a) 3
b) 2
c) 4
d) 5
Answer : c
40) In chlorobenzene, the -Cl group
a) activates the benzene ring more by resonace effect than deactivatng it by inductive ettect
b) deactivative
c)activates the benzene ring by resonace anddeactivates it by inductive etfect and both these effects are balanced
d) deactives the benzene ning by resonace and activates
it by inductive effect and both these effects are balanced.
Answer : b