Science Textbook Solutions for Class 8 Science Chapter 7 – Metals and Nonmetals
Important points to remember :
- Gold, silver, iron, copper, aluminium, magnesium, calcium, sodium, platinum are a few metals.
- Metals are good conductors of heat and electricity. Metals lose their valence electrons to produce positively charged ions, that is, cations.
- Some elements such as arsenic (As), Silicon (Si), Germanium (Ge), Antimony (Sb) have properties which are intermediate between metals and nonmetals. Such elements are called metalloids. Carbon, Sulphur, Phosphorus are a few nonmetals.
- Gold which is100 percent pure is called 24 carat gold. Pure gold is soft. As a result the ornaments made from pure gold bend or break due to pressure.
Question 1: Complete the table
Answer : Property of metal Use in every day life
i. Ductility – In electrical wires, cable wires etc.
ii. Malleability – Aluminium foil
iii. Conduction of heat – Cooking wares, microwave, electric press, straightening machine, electric belts
iv. Conduction of electricity – Bulb, tubelight, lamp, refrigerator, television
v. Sonority – Cymbals, doorbells
Question 2: Identify the odd term
A. Gold, silver, iron, diamond
Answer : Iron is odd one out because iron is non-lustrous in nature and others are lustrous.
B. Ductility, brittelness, sonority, malleability
Answer : Brittleness is odd one out because it is a property of non-metals and rest are the properties of metals.
C. Carbon, bromic, sulphur, phosphorus
Answer : Bromine is odd one out because it is liquid non-metal and others are solid non-metals.
D. Brass, bronze, iron, steel
Answer : Iron is odd one out because it is not an alloy and others are alloys.
Question 3: Write scientific reasons.
A. The stainless steel vessels in Kitchen have copper coating on the bottom.
Answer : The stainless steel vessels in Kitchen have copper coating on the bottom because copper is good conductor of heat and electricity, by using copper cookware, any meal can be prepared in a perfect and gentle way.
B. Copper and brass vessels are cleaned with lemon.
Answer : Citric acid in lemon juice etches away copper oxide that gives the vessels the dark color. When the oxide is removed, the copper metal underneath is exposed, it has a common ph range of 2-3 and reacts with metals and serves as a good cleaning fluid for metallic surfaces.
C. Sodium metal is kept in kerosene.
Answer : Sodium is a very reactive metal. It is kept in kerosene to prevent it from coming in contact with oxygen and moisture. If this happens, it will react with the moisture present in air and form sodium hydroxide which is a highly exothermic reaction.
Question 4: Answer the following
A. What is done to prevent corrosion of metals?
Answer : Metal corrosion occurs when metal is exposed to moisture and other elements or chemicals. While it is generally a natural process it can lead to a severe decrease in the functionality and esthetics of metal products. Whatever your reason for wanting to stop and prevent the corrosion of metals, here are some helpful ways to prevent corrosion of metals:
- Turn to non-corrosive metals such as aluminum and stainless steel.
- Keep the area around the metal surface dry.
- Use drying agents and moisture barrier products.
- Make sure underground piping is laid in a layer of backfill, such as limestone.
- Make sure any electrical components are cleaned regularly.
B. What are the metals that make the alloys brass and bronze ?
Answer : Brass is an alloy of copper and zinc. Bronze is a metal alloy consisting primarily of copper, usually with tin as the main additive, but sometimes with other elements such as phosphorus, manganese, aluminum, or silicon. Properties: Higher malleability than zinc or copper.
C. What are the adverse effects of corrosion ?
Answer : Some of the effects of corrosion include a significant deterioration of natural and historic monuments. Air pollution causes corrosion, and it’s becoming worse worldwide.
The Effects of Corrosion
- So what are the effects of corrosion that could actually affect your daily life?
- Vortex Energy Saver identifies some of the direct effects of corrosion, which include:
- Damage to commercial airplanes that could result in possible in-flight problems.
- Damage to oil pipelines that could cause a costly and dangerous rupture that creates significant environmental damage.
- Damage to bridge supports that could cause a bridge failure
- Release of harmful pollutants from iron corrosion that contaminates the air
- Costs of repairing or replacing household equipment that fails
D. What are use of Noble metals. ?
Answer : The noble metals are a group of metals that resist oxidation and corrosion in moist air. The noble metals are not easily attacked by acids. They are the opposite of the base metals, which more readily oxidize and corrode.
Uses of the Noble Metals
Generally speaking, the noble metals are used in jewelry, coinage, electrical applications, to make protective coatings, and as catalysts. The exact uses of the metals vary from one element to another. For the most part, these metals are expensive, so you might consider them “noble” because of their value.
Platinum, Gold, Silver, and Palladium: This are bullion metals, used to make coins and jewelry. These elements are also used in medicine, particularly silver, which is antibacterial. Because they are excellent conductors, these metals may be used to make contacts and electrodes. Platinum is an excellent catalyst. Palladium is used in dentistry, watches, spark plugs, surgical instruments, and as a catalyst.
Rhodium: Rhodium may be electroplated over platinum, sterling silver, and white gold to add shine and protection. The metal is used as a catalyst in the automotive and chemical industries. It’s an excellent electrical contact and can be used in neutron detectors.
Ruthenium: Ruthenium is used to strengthen other alloys, particularly those involving other noble metals. It’s used to make fountain pen tips, electrical contacts, and as a catalyst.
Iridium: Iridium is used in many of the same ways as ruthenium, as both metals are hard. Iridium is used in spark plugs, electrodes, crucibles, and pen nibs. It’s valued for making small machine parts and is an excellent catalyst.
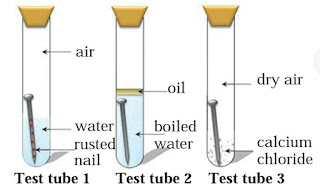
Question 5: Three experiments to study the process of rusting are given below. Observe the three test tubes and answer the following questions.
A. Why the nail in the test tube 2 is not rusted ?
Answer : Essential requirement for corrosion are:
Supply of oxygen Presence of water Material itself : The nail in the test tube 2 is not rusted because in test tube 2, we take boiling water. So, all the dissolved oxygen is removed from water and if the iron nail do not get supply of oxygen then corrosion is not carried out.
B. Why is the nail in the test tube 1 is rusted highly ?
Answer : The nail in the test tube 1 is rusted highly because in the test tube 1, iron nail meets with all the requirements which is essential for the process of corrosion. That’s Supply of oxygen in dissolved form, presence of water and material itself.
C. Would the nail in the test tube 3 get rusted ?
Answer : The nail in the test tube 3 is not rusted because calcium chloride is one of the best absorbent. So, it absorbs all the dissoved oxygen present in water. Hence corrosion process is not take place.
# Can you Tell
1. What are the three types in which the elements are generally classified?
Answer : These three groups are: metals, nonmetals, and inert gases. Let’s look at where these groups are located on the periodic table and correlate them with the ability to lose and gain electrons.
2. What are the metals and nonmetals that we use in everyday life?
Answer : The uses of metals and nonmetals are: Metals: Metals are used for making metal buildings. Iron is used for making automobiles, machinery, pipes, containers, nails, etc.
Non-metals: Hydrogen is used for the synthesis of ammonia and methyl alcohol, in welding torches, etc.
Tags : metals and non-metals class 8 worksheets pdf, metals and non metals class 8 ncert pdf, test questions on metals and non-metals, cbse class 8 science chapter 4 notes, class 8 science chapter 4 question answer in hindi, ncert solutions for class 8 science chapter 4 study rankers, assignment on metals and non-metals for class 8, metals and non-metals class 8 ppt