Maharashtra board class 10th science chapter 2 modern periodic table notes
Maharashtra state board Biology Textbook Notes for Class 10 are very important and crusial that helps the students in understanding the hard topics and helps them in the preparation of class 10 board examination as well as verious compititive entrance examinations also. Studying the answers to the questions in the Biology textbook will check your understanding of a particular topic and helps you determine your strengths and weaknesses.
Class 10 science part 1 textbook Notes for Class 10, science Chapter 2 modern periodic table Maharashtra Board Class 10 science chapter 2 modern periodic table Textbook Notes.
Maharashtra state board science Textbook Notes for Class 10 are very important and crusial that helps the students in understanding the hard topics and helps them in the preparation of class 10 board examination as well as verious compititive entrance examinations also. Studying the answers to the questions in the textbook will check your understanding of a particular topic and helps you determine your strengths and weak
Introduction-:
- In the universe 118 elements have been discovered till today.
- Classification of element-: is grouping of similar elements together and separating them from dis-similar ones.
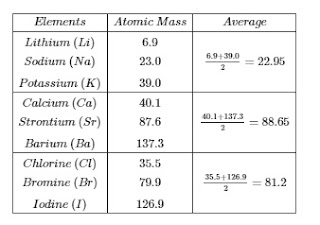
Dobereiners triads-:
- In the year 1817 a German scientist Dobereiners suggested that properties of elements are related to their atomic masses.
- He made groups of three elements each, having similar chemical properties and called them triads.
- Atomic mass of the second element of a triad is nearly equal to the arithmetic mean of atomic masses of other two elements.
- Elements in triad have similar properties.
Newland’s Law of Octaves -:
- Newland’s law of octaves Based on increasing atomic mass of elements
- It started with the lightest element hydrogen and ended up with thorium.
- He found that every eighth element had properties similar to those of first.
- .For example, sodium is the eighth element from lithium and both have similar properties.
Limitation -:
- Applicable only upto Calcium Properties of new elements couldn’t fit in it.
- In some cases properties of the elements were not same as defined by octave.
- eg: Fe was placed far away from Co and Ni Worked well only with lighter element
Mendeleev’s Periodic Table-:
- Dmitry Mendeleev a Russian chemist in 1869 gave Mendeleev’s Periodic Table.
- Till then 63 elements were known.
- Mendeleev arranged elements in increasing order of their atomic mass .He tried to put elements with similar properties in a group.
- Due to this we find empty boxes in his table.
Advantages of Mendeleev Periodic Table
- He left gap for some undiscovered elements. For Example, Eka Boron etc.
- This table also accommodate the noble gases
- Also corrected the atomic masses of certain elements.
Limitations of Mendeleev Periodic Table
- Position of isotopes cannot be explained.
- Position of hydrogen is not fixed. It is placed in group 1A, though its some properties matches with those of halogens.
Atomic Number -:
- Atomic number is defined as the total number of protons present in the nucleus of an atom.
- It is denoted by ‘Z’.Atoms of two different elements will always have different number of protons.
- Atoms of same element have same number of protons and thus they have same atomic number ‘Z’.
- In fact, elements are defined by the number of protons they possess. For hydrogen, Z = 1, because in hydrogen atom, only one proton is present in the nucleus.
Modern periodic law-:
- In 1913 A.D. the English scientist Henry Moseley demonstrated, with the help of the experiments done using X-ray tube, that the atomic number (Z) of an element corresponds to the positive charge on the nucleus or the number of the protons in the nucleus of the atom of that element .
- Modern periodic law states that the physical and chemical properties of elements are the periodic functions of their atomic number.
Structure of modern periodic table-:
- Elements are arranged in increasing oder.
- The vertical columns are known as groups and horizontal columns are known as periods; in the modern periodic table.
- There are 18 groups and 7 periods in the modern periodic table.
- Elements having same number of valence electrons are placed in the same group. For example; elements having valence electrons equal to 1 are placed in the 1st group, elements having valence electrons equal to 3 are placed in the 13th group, elements having valence electrons equal to 2 are placed in 2nd group except helium which is placed in 18th group, since it is an inert gas.
- Elements having same number of shells are placed in the same period.
Groups in Modern Periodic Table:
1st group: Alkali metals are placed in the 1st group in the modern periodic table. Hydrogen is also placed in the 1st group although hydrogen is not an alkali metal.
2nd group: Alkaline earth metals are placed in the 2nd group in the modern periodic table.
Elements placed in 1st and 2nd groups in the modern periodic table are collectively known as light metals.
3rd to 12th group: Transition elements are placed from 3rd to 12th group in the modern periodic table.
13th group: Metals are placed in the 13th group; except boron which is a metalloid.
14th group: Carbon, silicon, germanium, tin and lead are placed in this group. Among them, carbon is a non-metal, silicon and germanium are metalloids and tin and lead are metals.
15th group: Nitrogen, phosphorous, arsenic, antimony and bismuth are place in the 15th group; among which nitrogen and phosphorous are non-metals, arsenic and antimony are metalloids and bismuth is a metal.
16th group: Oxygen, sulphur, selenium, tellurium and polonium are placed in this group, among which oxygen, sulphur and selenium are non-metals, tellurium is metalloid and polonium is a metal.
17th group: Non-metals are placed in the 17th group. Since, halogens are placed in this group hence this group is also known as group of halogen.
18th group: Noble gases are placed in the 18th group. This group is also known as zero group.
Periods in Modern Periodic Table:
At present there are seven periods in the Modern Periodic Table.
1st period: This is known as very short period as there are only two elements, i.e. hydrogen and helium.
2nd and 3rd period: There are total 8 elements in each of the 2nd and 3rd periods. These periods are known as short periods.
4th and 5th period: There are total 18 elements in each of the 4th and 5th periods. These periods are known as long periods.
6th period: There are total 32 elements in 6th period. This period is known as very long period.
7th period : This period is known as incomplete period. Blank spaces in this period are supposed to be filled by the elements discovered in future. period: There are total 32 elements in 6th period. This period is known as very long period.
TRENDS IN THE MODERN PERIODIC TABLE:
Valency : No. of valence electrons present in the outermost shells.Valency remains the same down a group but changes across a period.
Atomic size : Atomic size refers to radius of an atom.Atomic size or radius decreases in moving from left to right along a period due to increase in nuclear chargeAtomic size increases down the group because new shells are being added as we go down the group.
Metallic Character – Ability of atom to lose the electron is known as Metallic Character. Metallic character decreases from left to right in a period. This is due to increase in nuclear charge. But non-metallic character increases left to right in a period. And metallic character increases down the group as the size increases it can easily lose electron.
Gradation in Halogen Family -:
The group 17 contains the members of the halogen family. All of them have the
general formula X2. A gradation is observed in their physical state down the group. Thus, fluorine (F2) and chlorine (Cl2) are gases, bromine (Br2 ) is a liquid while iodine (I2) is a solid.