Chemical Reactions and Equations Class 10 Questions Answers Maharashtra board Pdf
Maharashtra board class 10 Science Part 2 Chapter 3 Chemical Reactions and Equations textbook solutions:
- A chemical reaction is a process in which some substances undergo bond breaking and are transformed into new substances by formation of new bonds. The substances taking part in chemical reaction are called reactants, whereas the substances formed as a result of a chemical reaction by formation of new bonds are called products.
- In chemical reactions old bonds are broken and new ones are formed. Reaction often requires energy.
- Energy can be given in any form such as heat, light, electricity, or mechanical reactions in mechanical energy by shaking them to create sufficient contact between the molecules of the reagent, the atoms can be rearranged in a many ways.
- There are many examples of chemical reactions in daily life, such as digestion of food, making yogurt of milk, ripening of fruits, fermentation of grapes for making wine, fermentation of rice flour and lentils for making Idli or Dhokla and making food.
- If the number of atoms of the elements in the reactants in this equation is same as the number of atoms of those elements in the products. Such an equation is called a ‘balanced equation’.
- If the number of atoms of each element is not the same on the two sides of an equation, it is called an ‘unbalanced equation’.
- When two or more reactants combine in a reaction to form a single product, it is called a combination reaction.
- The chemical reaction in which two or more products are formed from a single reactant is called ‘‘Decomposition reaction”.
- The reaction in which the place of the ion of a less reactive element in a compound is taken by another more reactive element by formation of its own ions, is called displacement reaction.
- The reaction in which the ions in the reactants are exchanged to form a precipitate are called double displacement reactions
Maharashtra state board class 10th science part 1 chapter 3 chemical reaction and equation solutions:
(Oxidation, displacement, electrolysis, reduction, zinc, copper, double displacement, decomposition)
a. To prevent rusting, a layer of …….. metal is applied on iron sheets.
b. The conversion of ferrous sulphate to ferric sulphate is …….. reaction.
c. When electric current is passed through acidulated water …….. of water takes place.
d. Addition of an aqueous solution of ZnSO4 to an aqueous solution of BaCl2 is an example of ……. reaction.
Answer :
a. To prevent rusting, a layer of zinc metal is applied on iron sheets.
b. The conversion of ferrous sulphate to ferric sulphate is oxidation reaction.
c. When electric current is passed through acidulated water of electrolysis water takes place.
d. Addition of an aqueous solution of ZnSO4 to an aqueous solution of BaCl2 is an example of double displacement reaction.
a. What is the reaction called when oxidation and reduction take place simultaneously? Explain with one example
Oxidation is the loss of electrons or an increase in oxidation state by a molecule, atom, or ion.Reduction is the gain of electrons or a decrease in oxidation state by a molecule, atom, or ion.For example: If we add stannous chloride solution to the yellow solution of ferric chloride then light green ferrous chloride solution and stannic chloride solution are produced.
2FeCl3 (aq) + SnCI2 (aq) → 2FeCl2(aq) + SnCI4 (aq)2FeCl3 (aq) + SnCI2 (aq) → 2FeCl2(aq) + SnCI4 (aq)
Before the reaction, 3Cl atoms were attached to each iron atom. After the reaction, only two chlorine atoms are attached. That is one negative chlorine atom is released. Therefore, reduction of FeCl3 happened or this reactor on the other hand, before reaction two chlorine atoms where attached with each atom of tin (stannum). Due to the above reaction the number of chlorine attached to tin atom increases to four. That is, oxidation of SnCl2 has taken place. Therefore, in this reaction oxidation of one substance and reduction of another substance take place simultaneously. This is called redox reaction.
b. How can the rate of the chemical reaction, namely, decomposition of hydrogen peroxide be increased?
Answer : The rate of decomposition of hydrogen peroxide can be increased by having the reaction occurs in the presence of iodide ion. The reaction is proceed by two step mechanism:
Step 1:
H2O2 (aq) + I− (aq) → H2O (l) + IO− (aq) Step 2: IO− (aq) + H2O2 (aq)→ H2O (l)+ O2 (g)+ I− (aq)Step 1: H2O2 (aq) + I- (aq) → H2O (l) + IO- (aq) Step 2: IO- (aq) + H2O2 (aq)→ H2O (l)+ O2 (g)+ I- (aq)
c. Explain the term reactant and product giving examples.
Product: the substance which forms as a result of chemical reaction is called products.
The new substance produced as a result of chemical reaction is called products.
For example: When two sodium atoms react with two chlorine atoms(reactants), they give a completely new compound (product) i.e. sodium chloride (two atoms).
2Na+Cl2→2NaCl2Na+Cl2→2NaCl
d. Explain the types of reaction with reference to oxygen and hydrogen. Illustratre with examples
Answer : There are main three types of chemical reactions with reference to oxygen and hydrogen:
1.Combination Reaction
When two atoms react to form a compound, it is know as combination reaction.
For example: 2H2 + O2→ 2H2O2H2 + O2→ 2H2O
2. Decomposition Reaction
When a compound breaks into simple molecular substances from which it is made up of, it is know as decomposition reaction.
For example: 2H2O→ 2H2 + O22H2O→ 2H2 + O2
3. Oxidation and reduction reaction:
(i) The addition of oxygen to a substance is called oxidation.
(ii) The removal of hydrogen to a substance is called oxidation.
(i) The addition of hydrogen to a substance is called reduction.
(ii) The removal of oxygen to a substance is called reduction.
For example: CuO +H2→Cu + H2OCuO +H2→Cu + H2O
In the above reaction, copper oxide is changing to Cu. That is, oxygen is being removed from copper oxide. So, copper oxide is being reduced to copper.
In the above reaction, H2 is changing into H2O. That is , oxygen is being added to hydrogen. So, hydrogen is being oxidised to water.
e. Explain the similarity and difference in two events, namely adding NaOH to water and adding CaO to water.
1) NaOH (s) + H2O(l) →Na⁺(aq) + OH⁻(aq) + Δ(HEAT)
- Both of the equations are exothermic. It means a lot of heat is evolved during the reaction.
- Both reaction form strong basic solution.
Differences :
- Sodium Hydroxide is strong base dissociates to form Na⁺ and OH⁻ ion. While Calcium oxide added water to form Calcium Hydroxide which further dissociates.
- NaOH is a monoacidic base. and CaO is a Di-Acidic base.
- NaOH, CaO should be added to water gradually with constant stirring.CaO on reacting with water produces basic solution called as Calcium hydroxide which is used for white washing and this reaction is more dangerous as compare to NaOH.
a. Endothermic reaction
b. Combination reaction
c. Balanced equation
d. Displacement reaction
Answer :
Answer :
- An exothermic reaction is a chemical reaction that releases energy in the form of heat and light.
- It is the opposite of an endothermic reaction. Exothermic Reaction means “exo” meaning releases and “thermic” means heat.
- So the reaction in which there is release of heat with or without light is called exothermic reaction.
- Expressed in a chemical equation: reactants → products + energy.
- For example: Combustion is exothermic reaction.
- The chemical reaction between zinc granules and dilute sulphuric acid is exothermic reaction.
b. Combination reaction:
Answer : Those reactions in which two or more substances combine to form single substance is called combination reaction
For example: Magnesium and oxygen combine, when heated, to form magnesium oxide.
2Mg + O2 → 2MgO
c. Balanced equation:
Answer : A balanced equation is an equation for a chemical reaction in which the number of atoms for each element in the reaction and the total charge are the same for both the reactants and the products. In other words, the mass and the charge are balanced on both sides of the reaction.
- The balanced equation is: 2 Fe2O3 + 3 C → 4 Fe + 3 CO2
- Both the left and right sides of the equation have 4 Fe, 6 O, and 3 C atoms.
- When you balance equations, it’s a good idea to check your work by multiplying the subscript of each atom by the coefficient.
- When no subscript is cited, consider it to be 1. It’s also good practice to cite the state of matter of each reactant.
- This is listed in parentheses immediately following the compound. For example, the earlier reaction could be written as: 2 Fe2O3(s) + 3 C(s) → 4 Fe(s) + 3 CO2(g) where s indicates a solid and g is a gas state of matter.
d. Displacement reaction:
Answer : Displacement reaction is a chemical reaction in which a more reactive element displaces a less reactive element from its compound. Both metals and non-metals take part in displacement reactions.
Chemical reactivity of metals is linked with their relative positions in the activity series.
A metal placed higher in the activity series can displace the metal that occupies a lower position from the aqueous solution of its salt.
For example: zn (S) + Cuso4 (aq) —– Zn so4 (aq) + Cu (S)
Question 4: Give scientific reasons.
a.When the gas formed on heating limstone is passed through freshly prepared lime water, the lime water turns milky.
When carbon dioxide is passed through lime water, the Carbon dioxide reacts with calcium hydroxide to form Calcium carbonate which is a white precipitate, then lime water turns milky as there is formation of calcium carbonate.
CaCo3→ CaO +CO2CO2+ Ca(OH)2→CaCO3CaCo3→ CaO +CO2CO2+ Ca(OH)2→CaCO3
b. It takes time for pieces of Shahabad tile to disappear in HCl, but its powder disappears rapidly
for example: powdered salt react and water will dissolve, but salt rocks and water will not dissolve.
c. While preparing dilute sulphuric acid from concentrated sulphuric acid in the laboratory, the concentrated sulphuric acid is added slowly to water with constant stirring
d. It is reccommended to use air tight container for storing oil for long time.
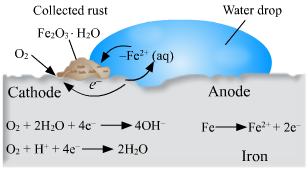
Question 5: Observe the following picture a write down the chemical reaction with explanation.

Answer : Rusting of iron is an oxidation process. The rust on iron does not form by simple reaction between oxygen and iron surface. The rust is formed by an electrochemical reaction.’ Fe’ oxidises to fe2O3.H2O (rust)on one part of iron surface while oxygen gets reduced to H2O in another part of surface.
1. Fe is oxidised to Fe2 ++ in the anode region.
Fe (S) gives Fe2+ (aq ) + 2 e-
2. O2 is reduced to form water in the cathode region .
O2(g) + 4 H+ (aq ) +4e- gives 2 H2O (l)
When Fe 2+ ions travel from the anode they react with water and further get oxidised to form Fe3+ ions. A reddish colored hydrated oxide is formed from Fe3+ ions it’s is called rust. Because of various components of atmosphere oxidation of metals takes place iron rust and reddish coloured layer is collected on it . This is corrosion of iron.
Question 6: Identify from the following reaction the reactants that undergo oxidation and reduction.
a. Fe + S → FeS
b. 2Ag2O → 4 Ag + O2 ↑
c. 2Mg + O2 → 2MgO
d. NiO + H2 → Ni + H2O
Answer :
a. Fe + S → FeS
In a reaction, Fe is changing to FeS. That means, iron loses electrons to form FeS. Loss of electron from a substance is called oxidation, so iron undergoes oxidation.
b. 2Ag2O → 4 Ag + O2 ↑
In a reaction, silver oxide is changing to silver. That is, oxygen is being removed from silver oxide. Removal of oxygen from substance is called reduction, so silver oxide undergoes reduction.
c. 2Mg + O2 → 2MgO
In a reaction, magnesium is changing to magnesium oxide. That means, oxygen is being added to magnesium. Addition of oxygen to a substance is called oxidation, so magnesium undergoes oxidation.
d. NiO + H2 → Ni + H2O
In a reaction, Nickle oxide is changing to nickle. That is, oxygen is being removed from nickle oxide. Removal of oxygen from substance is called reduction, so nickle oxide undergoes reduction.In a reaction, hydrogen is changing to H2O. That is,oxygen is being added to hydrogen. Addition of oxygen to a substance is called oxidation, so hydrogen undergoes oxidation.
Question 7: Balance the following equation stepwise :
a. H2S2O7(l) + H2O(l) → H2SO4(l)
Answer : Step 1. Count the number of each atom in reactant side:
H= 4
S=2
O=8
Step 2. Count the number of each atom in product side:
H= 2
S=1
O=4
Step 3.Then balance the number of each atom in an equation by multiplying reactant and product side with numeral value:
If we multiply product side by 2, then number of atoms in product and reactant side gets balance.
H2S2O7(l) + H2O(l) → 2H2SO4(l)
b. SO2(g) + H2S(aq) → S(s) + H2O(l)
Answer : Step 1. Count the number of each atom in reactant side:
H= 2
S=2
O=2
Step 2. Count the number of each atom in product side:
H= 2
S=1
O=2
Step 3.Then balance the number of each atom in an equation by multiplying reactant and product side with numeral value:
If we multiply H2S by 2 in the reactant side and S by 3 and H2O by 2 in the product side, then number of atoms in product and reactant side gets balance.
SO2(g) + 2H2S(aq) → 3S(s) + 2H2O(l)
c. Ag(s) + HCl(aq) → AgCl ↓+ H2 ↑
Answer : Step 1. Count the number of each atom in reactant side:
H= 1
Ag=1
Cl=1
Step 2. Count the number of each atom in product side:
H= 2
Ag=1
Cl=1
Step3.Then balance the number of each atom in an equation by multiplying reactant and product side with numeral value:
If we multiply Ag by 2 and HCl by 2 in the reactant side and AgCl by 2 in the product side, then number of atoms in product and reactant side gets balance.
2Ag(s) + 2HCl(aq) → 2AgCl ↓+ H2 ↑
d. NaOH(aq) + H2SO4(aq) → Na2SO4(aq) + H2O(l)
Answer : Step 1. Count the number of each atom in reactant side:
Na= 1
H=3
O=5
S=1
Step 2. Count the number of each atom in product side:
Na= 2
H=2
O=5
S=1
Step 3.Then balance the number of each atom in an equation by multiplying reactant and product side with numeral value:
If we multiply NaOH by 2 in the reactant side and H2O by 2 in the product side, then number of atoms in product and reactant side gets balance.
2NaOH(aq) + H2SO4(aq) → Na2SO4(aq) +2H2O(l)
Question 8: Identify the endothermic and exothermic reaction.
a. HCl + NaOH → NaCl + H2O + heat
Answer : Heat is released in the product side, as it mentioned in the above reaction. So, It is an exothermic reaction because heat is evolved in exothermic reaction.
b. 2KClO3(s)→Δ2KCl(s)+3O2↑⏐⏐2KClO3s→∆2KCls+3O2↑
Answer : Heat is given in the product side to break the compound into simpler substances, as it mentioned in the above reaction. So, It is an endothermic reaction because heat is supplied in exothermic reaction.
c. CaO + H2O → Ca(OH)2 + heat
Answer : Heat is released in the product side, as it mentioned in the above reaction. So, It is an exothermic reaction because heat is evolved in exothermic reaction.
d. CaCO3(s)→ΔCaO(s)+CO2↑⏐⏐CaCO3s→∆CaOs+CO2↑
Answer : Heat is provided in the product side to break the compound into simpler substances, as it mentioned in the above reaction. So, It is an endothermic reaction because heat is supplied in exothermic reaction.
Question 9: Match the columns in the following table :
Reactants | Products | Type of chemical reaction |
|
|
|
|
|
|
|
|
|
|
|
|
Answer :
| Reactants | Products | Type of chemical reaction |
| BaCl2(aq) + ZnSO4(aq) | BaSO4↓ + ZnCl2(aq) | Double displacement |
| 2AgCl(s) | 2Ag(s) + Cl2(g) | Decomposition |
| CuSO4(aq) + Fe(s) | FeSO4(aq) + Cu(s) | Displacement |
| H2O(l) + CO2(g) | H2CO3(aq) | Combination |