Major Difference between osmosis and diffusion Similarities
Distinguish between Osmosis and Diffusion :
Students are often asked to explain the similarities and differences between osmosis and diffusion or to compare and contrast the two forms of transport. To answer the question students need to know the definitions of osmosis and diffusion and really understand what it means. Osmosis and diffusion are the two different types of passive transport system occurs in Plants, which play a vital role in moving molecules in and out of the cell. Osmosis and diffusion are two related concepts whose differences and similarities can sometimes cause confusion. The cause of this confusion is the fact that both processes, osmosis and diffusion, are transport mechanisms that involve the movement of chemical substances following their concentration gradient. To clearly understand the 5 difference between osmosis and diffusion, let’s start with the definition of each of these concepts:
Read : Difference between Ligaments and Tendons
Defination of Osmosis :
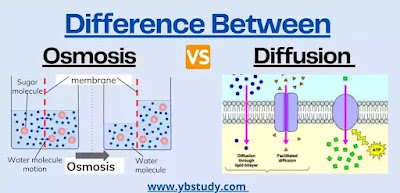
Osmosis is the movement of molecules of a solvent through a semi-permeable membrane from a dilute solution to a more concentrated solution . The osmosis process dilutes the more concentrated solution (since it receives more solvent) and concentrates the more dilute solution (since it loses solvent), so it stops when both concentrations become equal.
Osmosis occurs since the solvent is actually more concentrated in the diluted solution (less concentration of solute implies a higher concentration of solvent), so it moves towards the concentrated solution in which, actually, the solvent is more diluted . In other words, it follows its concentration gradient.
Examples of osmosis: Examples of this are red blood cells that swell when exposed to fresh water and plant root hairs that absorb water. Glue candy into the water to see an easy demonstration of osmosis. The gel of the candy acts as a semipermeable membrane.
Defination of Diffusion :
Diffusion is the movement of any type of particle from an area of high concentration to an area of lower concentration . The ultimate effect of diffusion is to equalize the concentrations in all available spaces to the particles.
In diffusion, the particles that diffuse can be from the small drops of an aerosol, as when we spread a perfume with a spray bottle, to molecules of solute or even of solvent in a solution.
Examples of diffusion: Examples of diffusion are the scent of perfume that fills an entire room and the movement of small molecules across a cell membrane. One of the simplest demonstrations of diffusion is to add a drop of food coloring to the water. Although other transport processes do occur, diffusion is the key player.
Read : Difference between Glycolysis and TCA Cycle
Similarities Between osmosis and diffusion :
Before we start listing the differences between osmosis and diffusion, let’s start by seeing how they are similar:
- Both biological transport processes are involved in the movement of particles.
- Both processes are passive processes.
- Both processes do not consume cellular energy molecules called “ATP”.
- Both osmosis and diffusion are very important for the survival of the cell.
- In both processes, the particles go from a high concentration to a lower concentration.
- In both diffusion and osmosis, the phenomenon of particle transport occurs due to the difference in concentrations in different areas of the container that contains them, and it is always in the direction from highest to lowest concentration.
- Both osmosis and diffusion equalize the concentration of two solutions.
- Both diffusion and osmosis are spontaneous processes, so they do not require an energy supply to occur.
- As the particles move along their concentration gradient, the difference in concentrations decreases, so both diffusion and osmosis stop when the concentration is the same everywhere.
The similarities Between osmosis and diffusion end here. Now, let’s look at the differences between diffusion and osmosis these two transport mechanisms.
| Osmosis | Diffusion |
|---|---|
| It only occurs between two liquid solutions. | It can occur in any medium, regardless of whether it is solid, liquid or gaseous. |
| It only involves the transport of solvent molecules, never solutes. | Involves the transport of any type of particle, including both solutes and solvents |
| Osmosis is a passive process. | Diffusion is also passive process. |
| Osmosis requires water for movement of Particles. | Diffusion does not require water for movement of Particles. |
| In Osmosis the flow of Particles occurs only in one direction. | In Diffusion the flow of particles occurs in All directions. |
| It involves the existence of two physically separate compartments or media. | It can occur between two separate media, as long as the spreading sparticles can cross the barrier that separates them, or it can occur within a single compartment. For example, within a solution, or in the air contained in a closed room. |
| It requires semi-permeable membrane (which only allows the passage of the solvent) that separates both compartments so that it can occur. | It occurs without semipermeable membranes or barriers of any kind. |
| Turgor and hydrostatic pressure oppose osmosis. | Hydrostatic pressure and turgor, in general, do not affect diffusion. |
| Osmosis occurs in only similar types solutions. | Diffusion occurs in both similar and different types of Solutions. |
| Osmosis depends mainly on the number of solute potential. | Diffusion Does not depend on solute potential, pressure potential, or water potential. |
| Osmosis does not helps in the uptake of minerals and nutrients | It helps in the uptake of minerals and nutrients |
| Osmosis depends on the total concentration of all solutes that are not able to cross the semipermeable membrane (osmotically active solutes). | Diffusion depends solely on the concentration of the solute or the particles that are diffusing. |
| The concentration of the solvent does not become equal on both sides of the membrane. | The concentration of the diffusion substance equalizes to fill the available space. |
| In biology, osmosis is only discussed in terms of the movement of water through the cell membrane. Other solvents are not considered. | In biology, diffusion is only discussed in terms of the movement of solutes across the cell membrane, through intracellular fluid, or through extracellular fluid. The movement of water through the membrane is not considered as diffusion, even though osmosis is a particular type of diffusion. |
Read : Difference between Mitosis and Meiosis
What is Osmosis?
Osmosis is a passive diffusion phenomenon that occurs when there are two solutions in a medium with different concentrations of solutes, which are separated by a semi-permeable membrane (allows only the solvent to pass). This phenomenon occurs spontaneously without the need for ATP energy intake.
There are three possible aqueous media separated by a semipermeable membrane :
- Hypotonic medium : the solute concentration is lower compared to the contiguous solution.
- Hypertonic medium : the solute concentration is higher compared to the contiguous solution.
- Isotonic medium (in equilibrium): when both solutions have the same concentration.
How is osmosis produced?
- During the osmosis the solvent diffuses from the solution of lower concentration (more dilute) to the solution of higher concentration through the semi-permeable membrane until the concentrations balance. This phenomenon occurs from the hypotonic media to the hypertonic media.
- The osmotic process can occur inside organisms or conversely in the external environment.
- Inside organisms for example, red blood cells can be in hypertonic solution. Thento equalize the concentration with the external environment, the red blood cells release water, becoming wrinkled and can cause death. On the contrary, when the dissolution is hypotonic, the red blood cells absorb water and swell, which can also cause the breakdown of the blood cell and its death (cell lysis).
- In the outdoor environment for example, those living organisms such as plants exposed to saline environments (high concentration solution) present high osmotic pressure, which means that they require an osmoregulation system to tolerate salinity.
- The osmotic pressure is the extra pressure necessary to stop the flow of the solvent through the semi-permeable membrane.
What is Diffusion ?
- Molecules, either in liquid state or gaseous state, exhibit constant, but random movement because of their inherent kinetic energy. The net movement of molecules is called diffusion.
- The direction of diffusion is concentration dependent, Irrespective of the kind and state, molecules move from higher concentration to lower concentration of the same kind. Greater the concentration gradient between two systems, greater is the rate of diffusion.
- In a system containing more than one kind of molecule, each one of them, find their own gradient. The rate of diffusion is governed by other factors like concentration gradient, temperature, density of molecules, pressure and medium through which it diffuses.
- The diffusipn of any substance across a membrane also depends on its solubility in lipids. Substances soluble in lipids diffuse through the membrane faster. Molecules can diffuse faster in gases and liquids than through solids.
- Movement by diffusion is passive and no energy expenditure takes place. Diffusion is a slow process and is not dependent on a living system.
- It can account for only short distance movement of molecules. For example, the movement of a molecule across a typical plant cell (about 50 um) takes approximately 2.5 secs.
- Substances that have a hydrophilic moiety find it difficult to pass through the membrane. Some membrane proteins provide sites at which such molecules can cross the membrane. This process is called facilitated diffusion.
- In facilitated diffusion, special proteins help move substances across membranes without expenditure of ATP