Download Class 12 Chemistry Syllabus Pdf 2025 Free
Class 12 Chemistry Syllabus: The Central Board of Secondary Education has unveiled the Mathematics (subject code 041) curriculum for class 12th students of the 2025 academic session. Comprising six units, the syllabus outlines a theory exam accounting for 80 marks. Unlike traditional chapter-wise divisions, the syllabus allocates weightage based on the competencies assessed by the questions. Students can access the complete syllabus contents and download the CBSE Class 12 Maths Syllabus 2025 PDF from this article.
To facilitate their studies, students can download the latest CBSE Class 12 Maths Syllabus PDF 2025 from the provided link. Familiarizing themselves with the syllabus will enable students to anticipate the topics they will encounter in Maths throughout the academic year.

It is recommended that students refer to the syllabus regularly during their study sessions. This CBSE Syllabus PDF serves as a valuable tool for students to track their progress by documenting the topics covered and those yet to be addressed.
The Higher Secondary stage of education is crucial as it introduces specialized discipline-based courses, particularly in subjects like Chemistry. Students choose Chemistry with the aim of pursuing careers in various fields such as medicine, engineering, technology, and applied areas of science and technology at the tertiary level. Therefore, it’s essential to provide learners with a strong conceptual foundation in Chemistry to prepare them for academic and professional challenges after this stage.
CBSE Class 12 Chemistry Syllabus 2025
The new and updated Class 12 Chemistry Syllabus is based on a disciplinary approach, ensuring rigor and depth while also ensuring that the Class 12 Chemistry Syllabus is not overly burdensome and remains comparable to international standards.
Over the past decade, the knowledge in Chemistry has evolved significantly, with emerging areas such as synthetic materials, biomolecules, natural resources, and industrial chemistry becoming increasingly important. These areas deserve inclusion in the Class 12 Chemistry Syllabus at the senior secondary stage.
Internationally, new formulations, nomenclature of elements and compounds, and standards for symbols and units of physical quantities are constantly evolving, as determined by scientific bodies like IUPAC and CGPM. It’s crucial to incorporate these updates into the Class 12 Chemistry Syllabus to ensure alignment with global standards.
The revised Class 12 Chemistry Syllabus addresses all these aspects by placing greater emphasis on the use of new nomenclature, symbols, and formulations, teaching fundamental concepts, applying concepts to industry and technology, logically sequencing units, and eliminating obsolete content and repetition.
| Sr. No | Unit Name | No. of Periods | Marks |
|---|---|---|---|
| I | Solutions | 10 | 7 |
| II | Electrochemistry | 12 | 9 |
| III | Chemical Kinetics | 10 | 7 |
| IV | d -and f -Block Elements | 12 | 7 |
| V | Coordination Compounds | 12 | 7 |
| VI | Haloalkanes and Haloarenes | 10 | 6 |
| VII | Alcohols, Phenols and Ethers | 10 | 6 |
| VIII | Aldehydes, Ketones and Carboxylic Acids | 10 | 8 |
| IX | Amines | 10 | 6 |
| X | Biomolecules | 12 | 7 |
| Total | 70 |
Class 12 Chemistry Syllabus Chapterwise and Important Points to Study
The Higher Secondary Stage of school education is pivotal because it introduces specialized discipline-based, content-oriented courses. Students embark on this stage after completing 10 years of general education, and many opt for Chemistry with the goal of pursuing careers in basic sciences or professional fields like medicine, engineering, and technology.
Additionally, they may choose to study applied areas of science and technology at the tertiary level. Therefore, it is essential to equip learners with a strong conceptual foundation in Chemistry during this stage. This foundation will enable them to confidently tackle the challenges posed by academic and professional courses beyond the higher secondary stage.
| Chapter | Important Points to Study |
|---|---|
| Chapter 1: Solid State | Types of solids and their properties – Crystal lattice and unit cell – Imperfections in solids – Electrical and magnetic properties of solids |
| Chapter 2: Solutions | Types of solutions – Solubility and factors affecting solubility – Raoult’s Law and colligative properties – Ideal and non-ideal solutions |
| Chapter 3: Electrochemistry | Electrochemical cells and their types – Galvanic cells and their working – Electrolytic cells and their applications – Conductance and its measurement |
| Chapter 4: Chemical Kinetics | Rate of a chemical reaction – Factors affecting the rate of reaction – Integrated rate equations – Arrhenius equation and activation energy |
| Chapter 5: Surface Chemistry | Adsorption and its types – Catalysis and its types – Colloids and their properties – Emulsions and their applications |
| Chapter 6: General Principles and Processes of Isolation of Elements | Principles of metallurgy – Extraction of metals – Refining of metals – Occurrence and extraction of non-metals |
| Chapter 7: The p-Block Elements | Group 15 elements: Nitrogen family – Group 16 elements: Oxygen family – Group 17 elements: Halogens – Group 18 elements: Noble gases |
| Chapter 8: The d- and f-Block Elements | Transition metals and their properties – Lanthanides and Actinides – Coordination compounds and their nomenclature – Isomerism in coordination compounds |
| Chapter 9: Coordination Compounds | Werner’s theory of coordination compounds – Ligands and coordination number – Bonding in coordination compounds – Applications of coordination compounds |
| Chapter 10: Haloalkanes and Haloarenes | Classification and nomenclature of halides – Preparation and properties of haloalkanes – Chemical reactions of haloalkanes – Environmental effects of haloalkanes |
| Chapter 11: Alcohols, Phenols and Ethers | Classification and nomenclature of alcohols, phenols, and ethers – Preparation and properties of alcohols, phenols, and ethers – Chemical reactions of alcohols, phenols, and ethers – Uses and applications of alcohols, phenols, and ethers |
| Chapter 12: Aldehydes, Ketones and Carboxylic Acids | Classification and nomenclature of aldehydes, ketones, and carboxylic acids – Preparation and properties of aldehydes, ketones, and carboxylic acids – Chemical reactions of aldehydes, ketones, and carboxylic acids – Uses and applications of aldehydes, ketones, and carboxylic acids |
| Chapter 13: Amines | Classification and nomenclature of amines – Preparation and properties of amines – Chemical reactions of amines – Uses and applications of amines |
| Chapter 14: Biomolecules | Carbohydrates: Classification, structure, and properties – Proteins: Structure, types, and functions – Enzymes: Types, mechanism of action, and applications – Nucleic acids: Structure, types, and functions |
| Chapter 15: Polymers | Classification and types of polymers – Preparation and properties of polymers – Biodegradable polymers – Uses and applications of polymers |
| Chapter 16: Chemistry in Everyday Life | Drugs and their classification – Chemicals in food – Cleansing agents – Role of chemistry in healthcare |
Download Class 12 Chemistry Syllabus
You can go through the Chemistry Class 12 Syllabus 2025 to understand the exam’s pattern which is both dynamic as well analytical in nature. Knowing your Syllabus is the first step in this three- stage exam journey and is also considered as screening stage.
Similar Articles
- Class 12 Physics Syllabus
- CBSE Class 12 Maths Syllabus 2025
- CBSE Class 12 Biolog Syllabus 2025
- Updated CBSE Class 11 Biology Syllabus
- CBSE Class 12 Biology Practical Syllabus 2025
- CBSE Class 11 Biology Practical Syllabus 2025
- NEET Syllabus for Beginners
Objectives of Class 12 Chemistry Syllabus
The objectives of the Chemistry curriculum at the Senior Secondary Stage are as follows:
- Promote understanding of basic facts and concepts in chemistry while maintaining the excitement of the subject.
- Prepare students to study chemistry in academic and professional courses at the tertiary level, such as medicine, engineering, and technology.
- Introduce students to emerging areas of chemistry and highlight their relevance in future studies and applications in various fields of chemical sciences and technology.
- Enable students to address challenges related to health, nutrition, environment, population, weather, industries, and agriculture using chemical knowledge.
- Develop problem-solving skills among students, fostering their ability to tackle complex issues.
- Familiarize students with industrial processes and their technological applications, providing practical insights.
- Demonstrate the interdisciplinary nature of chemistry by exploring its connections with other scientific disciplines such as physics, biology, geology, and engineering.
- Educate students on the different aspects of chemistry present in everyday life, enhancing their understanding of the subject’s practical implications.
- Cultivate an interest among students in studying chemistry as a discipline, encouraging further exploration and learning.
- Integrate life skills and values within the context of chemistry education, promoting holistic development and ethical decision-making.
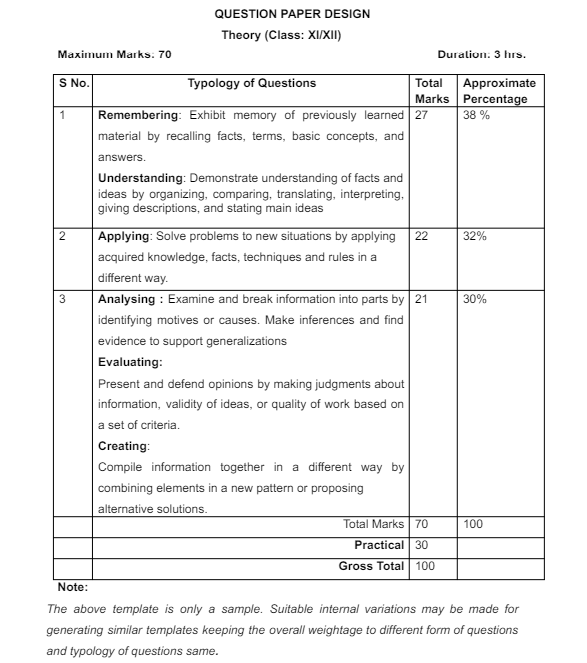
Class 12 Chemistry Question Paper Design